- Services
- Industries
- Automotive
- Battery
- Building inspection
- Fire alarms system testing
- Household appliances
- Installation materials
- Industrial machinery
- IT & audio video
- Laboratory, test & measurement
- Lighting equipment
- Maritime, oil & gas
- Medical & healthcare equipment
- Military & aerospace product testing
- Wireless & telecom
- Resources
- About
- Blog
- Events
November 30, 2020
Common Pitfalls in Medical Device Approvals: How to Overcome Them
Written by: Geir Olav Wang
Our experience in working with medical device manufacturers around the world gives us a window on the amazing innovation taking place in the healthcare industry today. Unfortunately, we also encounter device manufacturers who face one or more challenges in navigating the regulatory approval process. For many, these challenges often result in delays in bringing new and innovative devices to market, and can even lead to significant setbacks that threaten the manufacturer’s long-term viability.
Fortunately, most of those challenges are predictable, and device manufacturers can take steps to minimize their risk. Here’s a rundown of the regulatory pitfalls we see most often, and what manufacturers can do to reduce the risks associated with them.
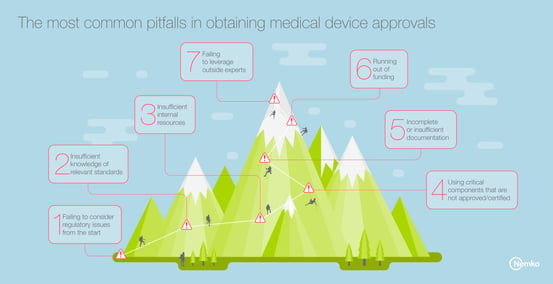
1. Failing to consider regulatory issues from the start
Many product development teams wait until the end of the device design and evaluation process before accounting for regulatory compliance issues. This results in additional time and investment to redesign non-compliant products, and can impact projected sales revenue. The solution? Make regulatory issues a key consideration through the product development process.
2. Insufficient knowledge of relevant standards
Key international standards, such as IEC/EN 60601 and ISO 14971, address device safety issues and the risk management process required of all device manufacturers. Without understanding the requirements of these standards, manufacturers will design products that fall short of minimum requirements. The solution? Read the standards, and understand how the requirements apply to your device.
3. Insufficient internal resources
The regulatory review and approval process takes time and requires dedicated resources to successfully navigate the process. But many teams minimize the time or the work required to address this essential aspect. The solution? Build your product development plan to account for the time and budget required to complete this process.
4. Using critical components that are not approved/certified
Another pitfall we frequently observe is the failure to design medical devices using critical components that have been pre-certified or otherwise approved for use. This typically results in the need for expanded testing with the accompanying increase in costs. The solution? Use only certified or other approved components in your device design.
5. Incomplete or insufficient documentation
Documentation is a critical element of the regulatory review and approval process. But few development teams focus on the importance of generating detailed documentation on product design, evaluation and pre-certification testing, which are required for product certification. The solution? Set rigorous internal documentation requirements covering the entire span of the product development process.

6. Running out of funding
Regulatory compliance testing and approval costs money. And the amount of funding required only increases when a manufacturer is seeking to gain widespread global access for their device and will need approval from regulators in the EU, the U.S. and other key markets. The solution? Be sure to build expenses specific to the regulatory compliance approval process into your budget from the start to avoid surprises.
7. Failing to leverage outside experts
Finally, few medical device manufacturers, no matter how competent or experienced, have the depth of knowledge or requisite expertise required to successfully navigate on their own the multi-faceted and ever-changing regulatory approval process required for global access. The solution? Find a trusted, independent third-party testing organization or advisory that can guide you through the process and provide the critical expertise necessary to achieve success.
By anticipating these potential pitfalls and proactively addressing them, device manufacturers can streamline the product approval process, save time and money, and reduce the risk of delays and setbacks in introducing innovative medical device technologies throughout the global marketplace.
For more information, please watch our on-demand video which will give you an introduction to the regulatory requirements for medical devices. You can also read our 10 steps guide for how you can achieve medical device approval.
Geir Olav Wang
Geir Olav Wang is the Sales Manager at Nemko Scandinavia and has worked with companies in the medical sector for over ten years. Together with our engineers, he has helped companies in Europe and Asia with testing and certification for worldwide market access. Before joining Nemko, he was Deputy Director Technical...
